
Recent Posts

News, Child Health
Bringing Home Baby: A Parents’ Guide to Making Things Safe and Sound

News
What Does it Mean When You Receive a Cancer Diagnosis?

News, Child Health
Empowering Children: How to Teach Them about Boundaries and Unwanted Touch

News, Child Health
Game On: Safeguarding Young Athletes from Concussions and Injuries

News, Women's Health
Atrium Health’s Female Cardiologists Lead the Way in Improving Women’s Heart Health

News, Child Health
Under pressure: Helping your child or teen cope with school-related stress

Your Health
Why Are So Many People Going Alcohol-Free
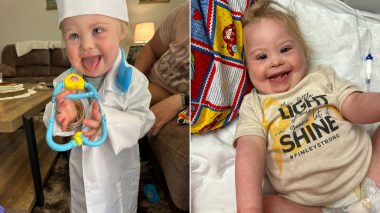
Child Health
Tiny Warrior Triumphs: Two-Year-Old’s Journey Through Open Heart Surgery and Leukemia Treatment
